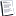 Re: IUPAC Nomenclature: SUCRALOSE
Re: IUPAC Nomenclature: SUCRALOSE
Assuming you have some carbohydrate knowledge here-
The blue ring would be galactose (if Cl were replaced by OH), hence galactopyranoside. The red ring would be fructose.
In a normal aldose, carbon 1 is the aldehyde carbon. In a normal ketose, carbon 1 is the carbon on the end nearest the carbonyl carbon. In this disaccharide, each sugar unit is numbered individually. So on the blue ring, carbon 1 is the rightmost carbon, since it's the one that started as an aldehyde (and is now an acetal). Counting around, the Cl is obviously on carbon 4. Alpha means that the acetal linkage (the bond to the red O) is on the opposite side of the ring as Carbon 6. Since Carbon 6 is in the equatorial position, it's "on top" of the ring (the H there is axial-down), and the acetal linkage is on the bottom.
Looking at the red ring, the linked formerly-carbonyl carbon is C2, so the topmost carbon is C1, and the other Cl is on C6. This ring is beta because the ketal linkage is on the bottom of the ring, as is C6 (on the far right). Same side = beta.

|

